Paenibacillus dendritiformis
| Paenibacillus dendritiformis | |
|---|---|
| |
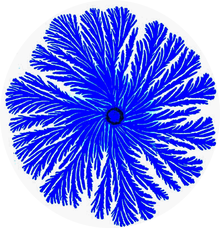
| Figure 1: A colony generated by the Branching (Tip splitting) morphotype bacteria of P. dendritiformis. The colony diameter is 6cm and the colors indicate the bacteria density (darker shade for higher density). | |
| Scientific classification | |
| Domain: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | |
| Species: | P. stellifer
|
| Binomial name | |
| Paenibacillus stellifer (Ash et al., 1994)
| |
Paenibacillus dendritiformis is a species of pattern-forming bacteria, first discovered in the early 90s by Eshel Ben-Jacob's group.[1][2] It is a social microorganism that forms colonies with complex and dynamic architectures. The genus Paenibacillus comprises facultative anaerobic, endospore-forming bacteria originally included within the genus Bacillus and then reclassified as a separate genus in 1993.[3] Bacteria belonging to this genus have been detected in a variety of environments such as: soil, water, rhizosphere, vegetable matter, forage and insect larvae.[4][5][6][7]
Paenibacillus spp.
[edit]In recent years there is an increasing interest in studies of Paenibacillus spp. since many were found to be important for industrial, agricultural and medical applications. These bacteria produce various extracellular enzymes such as polysaccharide-degrading enzymes and proteases, which can catalyze a wide variety of synthetic reactions in fields ranging from cosmetics to biofuel production.[8][9][10] Various Paenibacillus spp. also produce antimicrobial substances that affect a wide spectrum of micro-organisms such as fungi, soil bacteria, plant pathogenic bacteria and even important anaerobic pathogens as Clostridium botulinium.[11][12][13]
Pattern formation, self-organization and social behaviors
[edit]P. dendritiformis is a social microorganism: when grown under growth conditions that mimic natural environments such as hard surfaces, it forms colonies of 109-1012 cells with remarkably complex and dynamic architectures (Figure 1).[2][14][15] Being part of a large cooperative, the bacteria can better compete for food resources and be protected against antibacterial assaults.[14][15] The P. dendritiformis exhibit many distinct physiological and genetic traits including β-galactosidase-like activity causing colonies to turn blue on X-gal plates and multiple drug resistance (MDR) (including septrin, penicillin, kanamycin, chloramphenicol, ampicillin, tetracycline, spectinomycin, streptomycin and mitomycin C. Colonies that are grown on surfaces in Petri dishes exhibit several folds higher drug resistance in comparison to growth in liquid media. This particular resistance is believed to be due to a surfactant-like liquid front that actually forms a particular pattern on the Petri plate.
Similar to other social bacteria Paenibacillus species, P. dendritiformis can form complex patterns on semi-solid surfaces. Development of such complex colonies require self-organization and cooperative behavior of individual cells while employing sophisticated chemical communication.[14][15][16][17][18] Pattern formation and self-organization in microbial systems is an intriguing phenomenon, reflection social behaviors of bacteria[17][19] that might provide insights into the evolutionary development of the collective action of cells in higher organisms.[20][21][22][23][24]
P. dendritiformis colonies behave much like a multi-cellular organism, with cell differentiation and task distribution.[17][18][20][24] Accomplishing such intricate cooperative ventures requires sophisticated cell-cell communication[14][16][17][22][25] including semantic and pragmatic aspects of linguistics.[17]
Communicating with each other using a variety of chemical signals, bacteria exchange information regarding population size, a myriad of individual environmental measurements at different locations, their internal states and their phenotypic and epigenetic adjustments. The bacteria collectively sense the environment and execute distributed information processing to glean and assess relevant information. The information is then used by the bacteria for reshaping the colony while redistributing tasks and cell epigenetic differentiations, for collective decision-making and for turning on and off defense and offense mechanisms needed to thrive in competitive environments, faculties that can be perceived as social intelligence of bacteria.[17]
Morphotype transition
[edit]The P. dendritiformis, poses an intriguing collective faculty – the ability to switch between different morphotypes[14][15][26] to better adapt in complex environments. Mostly studied is the transition between the Branching (or tip-splitting) morphotype (Figure 1) and the Chiral morphotype (Figure 2) that is marked by curly branches with well defined handedness.
The morphotype transition (Figure 3), can be viewed as an identity switching[14][15][26][27][28] – the calls can cooperatively make drastic alterations of their internal genomic state, effectively transforming themselves into differently looking and behaving cells which can generate colonies with entirely different organization. Under conditions somewhat more favorable to motion, such as growth on a softer substrate, the bacteria engineer classes of chiral colony patterns in which the branches are thinner and curl in the same direction (Figure 2). Accompanying the colonial structure is a designed genome change: the bacteria are now programmed to become longer and have multiple chromosomes. The morphotype transition are both inheritable - the identity is maintained during LB growth and even through sporulation/germination, and reversible – for example the reverse transitions from chiral to ordinary branching occur on harder substrates (when higher bacteria densities are required to produce sufficient amounts of lubrication). Optical microscope observations during colony development reveal the following: upon elongation, the cells alter their collective movement from the typical run-and-tumble to a coordinated forward-backward movement with limited tumbling.
Genome sequence
[edit]The genome sequence of the P. dendritiformis can be downloaded on the NCBI website here, or genetic information can be received upon request from the Tauber Sequencing Initiative at Tel-Aviv University, Israel. The genome was sequenced by a hybrid approach using 454 Life Sciences and Illumina, achieving a total of 340X coverage, with 99.8% sequence identity between the two methods. Preliminary analysis of the P. dendritiformis genome (approximate size of 6.6Mbp) revealed 6,782 open reading frames (ORFs). The analysis also unveiled the P. dendritiformis potential to produces a wealth of enzymes and proteases as well as a great variety of antimicrobial substances that affect a wide range of microorganisms. The possession of these advanced defense and offense strategies render P. dendritiformis as a rich source of useful genes for agricultural, medical, industrial and biofuel applications.
Competition between sibling bacterial colonies
[edit]In 2000 it was discovered, that two sibling colonies (colonies taken from the same mother colony or from the same LB growth) of the P. dendritiformis inoculated side by side can inhibit the growth of one another (Figure 4).[28] Recent detailed studies of the phenomenon in the branching morphotype, revealed that the two colonies not only inhibit each other from growing into the territory between them but induced the death of those cells close to the border. Material extracted from the agar gel between two colonies was found to kill single growing colonies.[29] By employing molecular biology methods combined with the new genome sequencing information and bioinformatics, they discovered a new toxin (sibling lethal factor), which acts selectively only on the same bacterial strain. The findings suggest a new strategy for fighting bacteria by self-toxins they produce.[30]
See also
[edit]References
[edit]- ^ Ben-Jacob E, Schochet O, Tenenbaum A, Cohen I, Czirok A, Vicsek T. Generic modelling of cooperative growth patterns in bacterial colonies. Nature. Mar 3 1994;368(6466):46-49.
- ^ a b Ben-Jacob E, Shochet O, Tenenbaum A, Avidan O. Evolution of complexity during growth of bacterial colonies. Paper presented at: NATO Advanced Research Workshop, 1995; Santa Fe, USA.
- ^ Ash C, Priest FG, Collins MD. Molecular identification of rRNA group 3 bacilli (Ash, Farrow, Wallbanks and Collins). Proposal for the creation of a new genus Paenibacillus. Antonie van Leeuwenhoek. 1993;64(3-4):253-260.
- ^ Lal S, Tabacchioni S: Ecology and biotechnological potential of Paenibacillus polymyxa: a minireview. Indian J Microbiol 2009, 49:2-10.
- ^ McSpadden Gardener BB: Ecology of Bacillus and Paenibacillus spp. in Agricultural Systems. Phytopathology 2004, 94:1252-1258.
- ^ Montes MJ, Mercade E, Bozal N, Guinea J: Paenibacillus antarcticus sp. nov., a novel psychrotolerant organism from the Antarctic environment. Int J Syst Evol Microbiol 2004, 54:1521-1526.
- ^ Ouyang J, Pei Z, Lutwick L, Dalal S, Yang L, Cassai N, Sandhu K, Hanna B, Wieczorek RL, Bluth M, Pincus MR: Case report: Paenibacillus thiaminolyticus: a new cause of human infection, inducing bacteremia in a patient on hemodialysis. Ann Clin Lab Sci 2008, 38:393-400.
- ^ Konishi J, Maruhashi K: 2-(2'-Hydroxyphenyl)benzene sulfinate desulfinase from the thermophilic desulfurizing bacterium Paenibacillus sp. strain A11-2: purification and characterization. Appl Microbiol Biotechnol 2003, 62:356-361.
- ^ Raza W, Yang W, Shen QR: Paenibacillus polymyxa: Antibiotics, Hydrolytic Enzymes and Hazard Assessment. J Plant Pathol 2008, 90:419-430.
- ^ Watanapokasin RY, Boonyakamol A, Sukseree S, Krajarng A, Sophonnithiprasert T, Kanso S, Imai T: Hydrogen production and anaerobic decolorization of wastewater containing Reactive Blue 4 by a bacterial consortium of Salmonella subterranea and Paenibacillus polymyxa. Biodegradation 2009, 20:411-418.
- ^ Dijksterhuis J, Sanders M, Gorris LG, Smid EJ: Antibiosis plays a role in the context of direct interaction during antagonism of Paenibacillus polymyxa towards Fusarium oxysporum. J Appl Microbiol 1999, 86:13-21.
- ^ Girardin H, Albagnac C, Dargaignaratz C, Nguyen-The C, Carlin F: Antimicrobial activity of foodborne Paenibacillus and Bacillus spp. against Clostridium botulinum. J Food Prot 2002, 65:806-813.
- ^ von der Weid I, Alviano DS, Santos AL, Soares RM, Alviano CS, Seldin L: Antimicrobial activity of Paenibacillus peoriae strain NRRL BD-62 against a broad spectrum of phytopathogenic bacteria and fungi. J Appl Microbiol 2003, 95:1143-1151.
- ^ a b c d e f Ben-Jacob E. Bacterial self-organization: co-enhancement of complexification and adaptability in a dynamic environment. Phil. Trans. R. Soc. Lond. A. 2003;361(1807):1283-1312.
- ^ a b c d e Ben-Jacob E, Cohen I, Gutnick DL. Cooperative organization of bacterial colonies: from genotype to morphotype. Annu Rev Microbiol. 1998;52:779-806.
- ^ a b Bassler BL, Losick R: Bacterially speaking. Cell 2006, 125:237-246.
- ^ a b c d e f Ben-Jacob E, Becker I, Shapira Y, Levine H: Bacterial linguistic communication and social intelligence. Trends Microbiol 2004, 12:366-372.
- ^ a b Dunny GM, Brickman TJ, Dworkin M: Multicellular behavior in bacteria: communication, cooperation, competition and cheating. Bioessays 2008, 30:296-298.
- ^ Galperin MY, Gomelsky M: Bacterial Signal Transduction Modules: from Genomics to Biology. ASM News 2005, 71:326-333.
- ^ a b Aguilar C, Vlamakis H, Losick R, Kolter R: Thinking about Bacillus subtilis as a multicellular organism. Curr Opin Microbiol 2007, 10:638-643.
- ^ Dwyer DJ, Kohanski MA, Collins JJ: Networking opportunities for bacteria. Cell 2008, 135:1153-1156.
- ^ a b Kolter R, Greenberg EP: Microbial sciences: the superficial life of microbes. Nature 2006, 441:300-302.
- ^ Shapiro JA: Thinking about bacterial populations as multicellular organisms. Annu Rev Microbiol 1998, 52:81-104.
- ^ a b Shapiro JA, Dworkin M: Bacteria as multicellular organisms. 1st edn: Oxford University Press, USA; 1997.
- ^ Bischofs IB, Hug JA, Liu AW, Wolf DM, Arkin AP. Complexity in bacterial cell-cell communication: quorum signal integration and subpopulation signaling in the Bacillus subtilis phosphorelay. Proc Natl Acad Sci U S A. Apr 21 2009;106(16):6459-6464.
- ^ a b Ben-Jacob E, Cohen I. Cooperative formation of bacterial patterns. In: Shapiro JA, Dworkin M, eds. Bacteria as Multicellular Organisms New York: Oxford University Press; 1997:394-416.
- ^ Ben-Jacob E, Levine H. Self-engineering capabilities of bacteria. J R Soc Interface. 2005;3(6):197-214.
- ^ a b Ben-Jacob E, Cohen I, Golding I, et al. Bacterial cooperative organization under antibiotic stress. Physica A. 2000;282(1-2):247-282.
- ^ Be'er A, Zhang HP, Florin EL, Payne SM, Ben-Jacob E, Swinney HL. Deadly competition between sibling bacterial colonies. Proc Natl Acad Sci U S A. Jan 13 2009;106(2):428-433
- ^ Be'er A, Ariel G, Kalisman O, et al. Lethal protein produced in response to competition between sibling bacterial colonies. Proc Natl Acad Sci U S A. Apr 6 2010;107(14):6258-6263
External links
[edit]- Prof. Eshel Ben-Jacob's home page
- Realizing Social Intelligence of Bacteria
- Bacterial art
- Bacterial self–organization: co–enhancement of complexification and adaptability in a dynamic environment
- Bacterial linguistic communication and social intelligence
- The genius of bacteria
- Gambling on Bacteria
- The tree of Life: IQ Test for Bacteria
- Type strain of Paenibacillus dendritiformis at BacDive - the Bacterial Diversity Metadatabase
